中文 | English | Manager Zhang:13910082894(WeChat ID same) Tel:010-69293631 QQ:1918474247
Fluorescent Dyes、Calixarenes、Cyanine Laser Dyes、Nucleosides、Hair Intermediates、Insect Pheromones

On Wechat
The Relationship Between Judgment on Conformations of Calix[4]arenes and Their 1HNMR
2025-03-24
In 1942, Austrian chemist Zinke used para-substituted phenol for the reaction when studying a new type of phenolic resin, in order to obtain a new linear structure phenolic resin, he reacted p-tert-butylphenol and formaldehyde aqueous solution with sodium hydroxide as catalysis, and accidentally obtained a crystalline compound with a high melting point, and found that the product was not the expected linear structure, but a cyclic tetramer. More than 30 years later, the American chemist Gutsche C. D. made an in-depth study of this type of compound, and named it calixarene according to its structure similar to the Greek Holy Grail (claix originally refers to the Greek Holy Grail, arene refers to aromatic hydrocarbons, the Chinese translation was first proposed by Academician Huang Zhitang, Chinese Academy of Sciences, as shown in Figure 1 ), the cavity size of calixarenes can be adjusted by changing the number of phenol units in the skeleton, series calixarenes have been synthesized from calix[4]arenes to calix[20]arenes.
Figure 1: Image and positional naming of calixarene compounds
Note: This image is a reference to https://lifechemicals.com/contract-research/custom-synthesis/task-specific-calixarenes,and it is not authorized by the author. Thanks and apologies are hereby given without the author's consent.
calixarenes, like crown ethers and cyclodextrins, belong to macrocyclic organic compounds, and because their cavity size can be adjusted according to the number of repeating units, the conformation is flexible and changeable, and there are many modification sites along the upper and lower edges, which has made it a hot spot in research for half a century. It is known as the third generation of supramolecular host molecules following crown ether and cyclodextrin.
Due to the free rotation of the σ bond between the phenol unit and the bonded methylene, the hydroxyl group on the lower edge of the calixarenes can be circumferentially linked, which changes the conformation, and the calixarenes often exist in a mixture of multiple conformations in the solution. However, when the para-steric hindrance of the hydroxyl group is relatively large, for example, the p-tert-butyl calixarenes, the tert-pentyl calixarenes in the solid state or even in the solution, the cup shape shown in the above picture is real, and its four hydroxyl groups are at the bottom of the "cup", and they rely on each other to form a cyclic hydrogen bond to stabilize the shape of the "cup".
Gutsche refers to this stable conformation as the "cone" conformation. The other possible conformations are called partial cone, 1,2-alternate, and 1,3-alternate conformations. Therefore, whether calixarenes can truly form a cup-shaped conformation requires a specific analysis of specific calixarene, as shown in Figure 2.
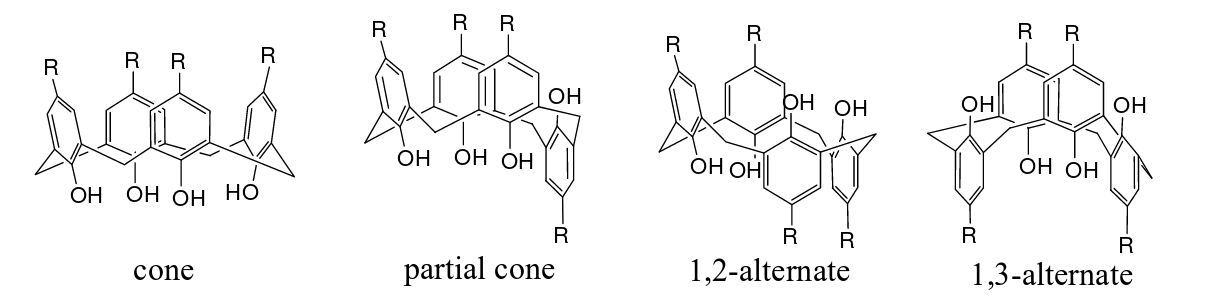
Figure 2: Different images of calixarene compounds
For example:
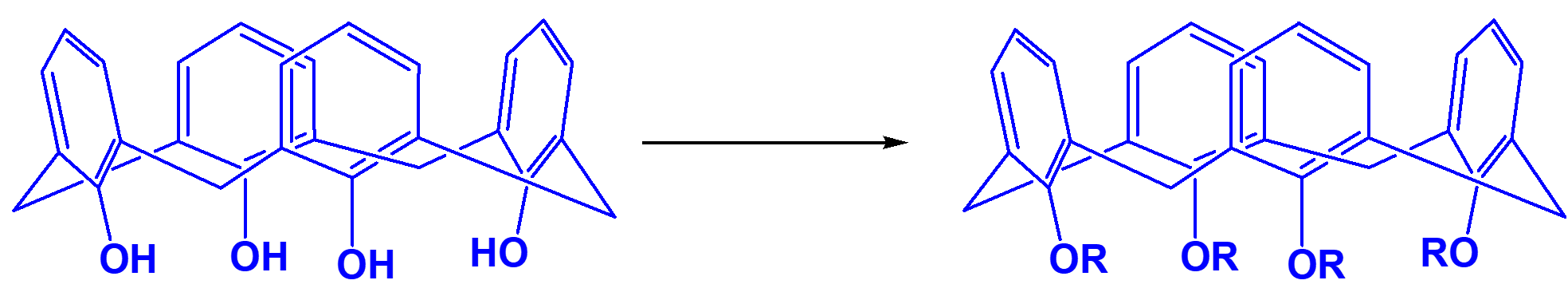
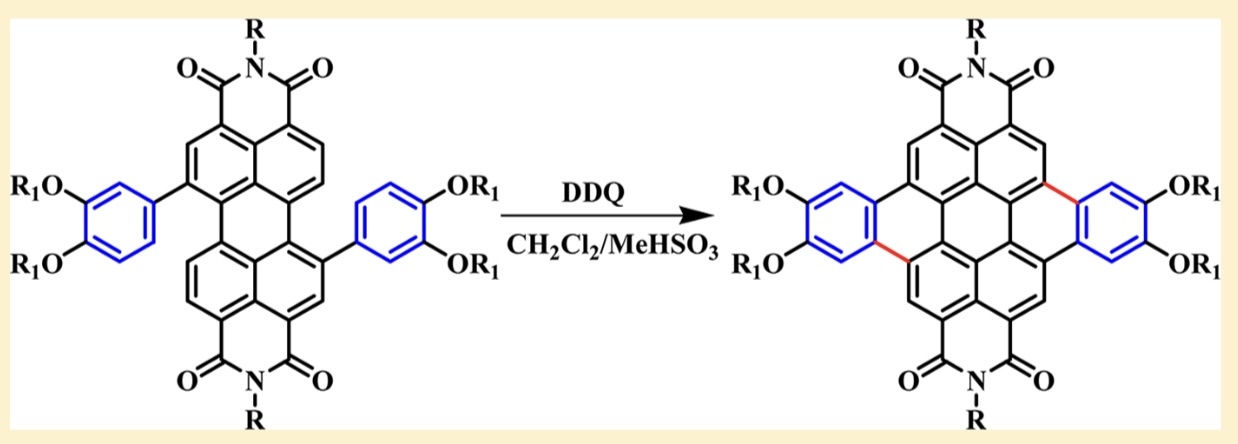
Figure 3: Etherification reaction of calix[4]arene compounds
When the hydroxyl group of calix[4]arene is etherified (see Fig. 3), the cup shape can be maintained when R is n-propyl, but not after methyl etherification, probably because the size of the etherification group also affects whether the alkoxy group can freely cross the cup ring to form a partial cone, 1,2-alternate (1,2-alternate) and 1,3-alternate (1,3-alternate) conformation.
So, how do we know what kind of conformation does a calixarene actually exist? There are many methods, one is that the conformation can be directly "seen" according to the quaternary X-diffraction measurement, but it is very difficult to cultivate single crystals that meet the requirements. Such as compound 10 in Fig. 4.
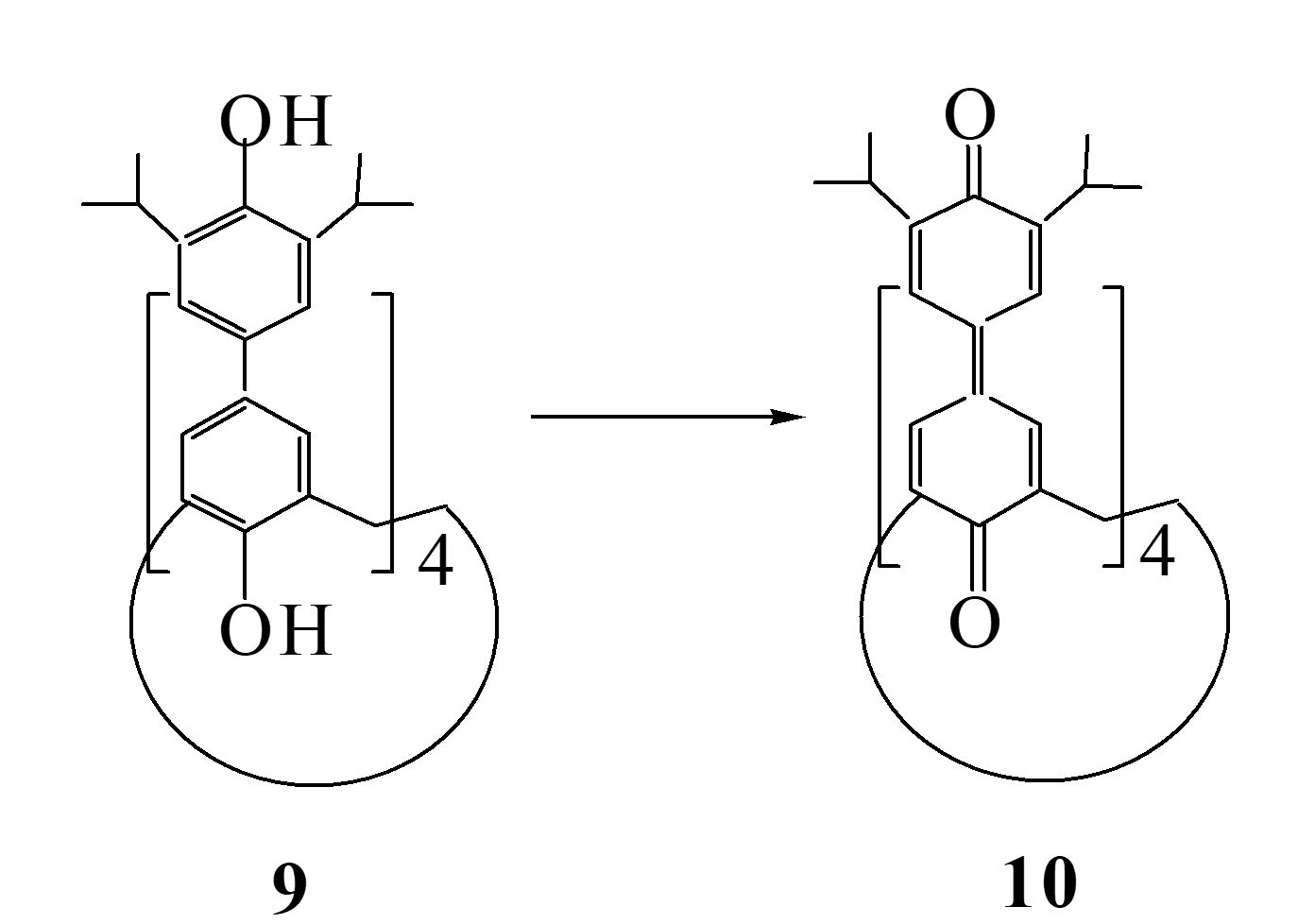
Figure 4: Oxidation of calixarene compound 9 to benzoquinone
By four-circle diffractometer compound 10 can clearly see that its cup-like structure is 1,3 alternating type, and it can also be seen that the "cup" is really filled with water (molecules)🙂。
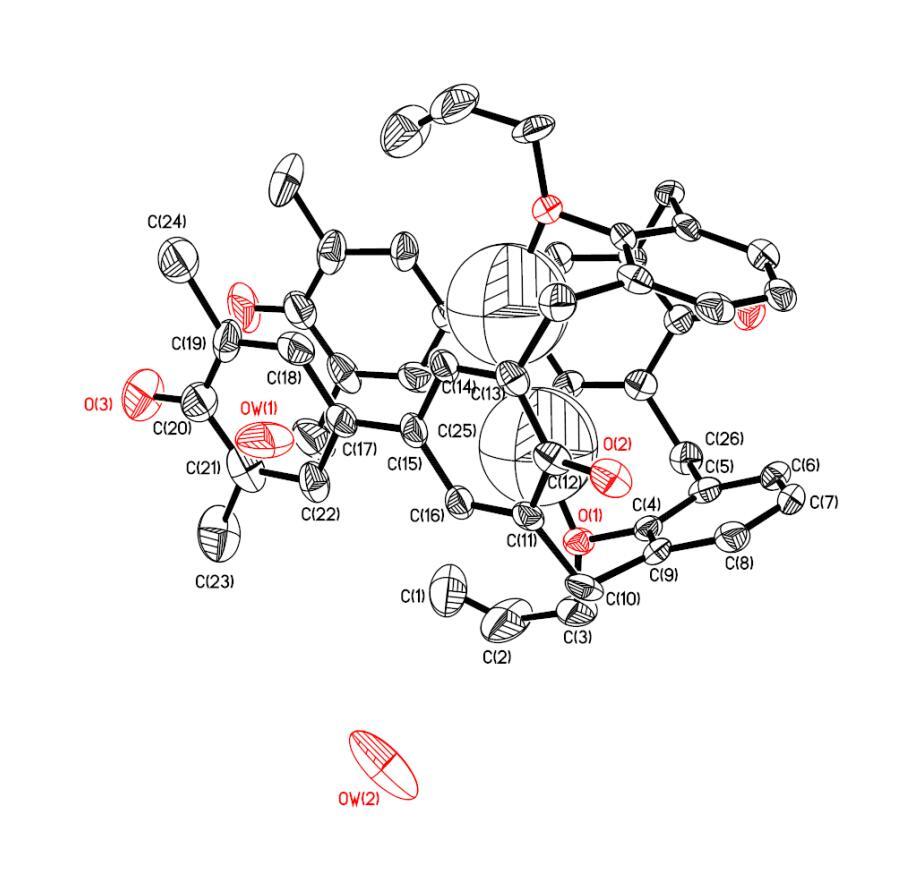
Figure 5: Four-element X-ray diffraction of calixarene compound 10 reflects its 1,3-conformation
Another inexpensive and simple approach is to study the tert-butyl calix[4]arene in solution predominantly in a conical conformation, as shown in Figure 6, based on dynamic NMR studies.
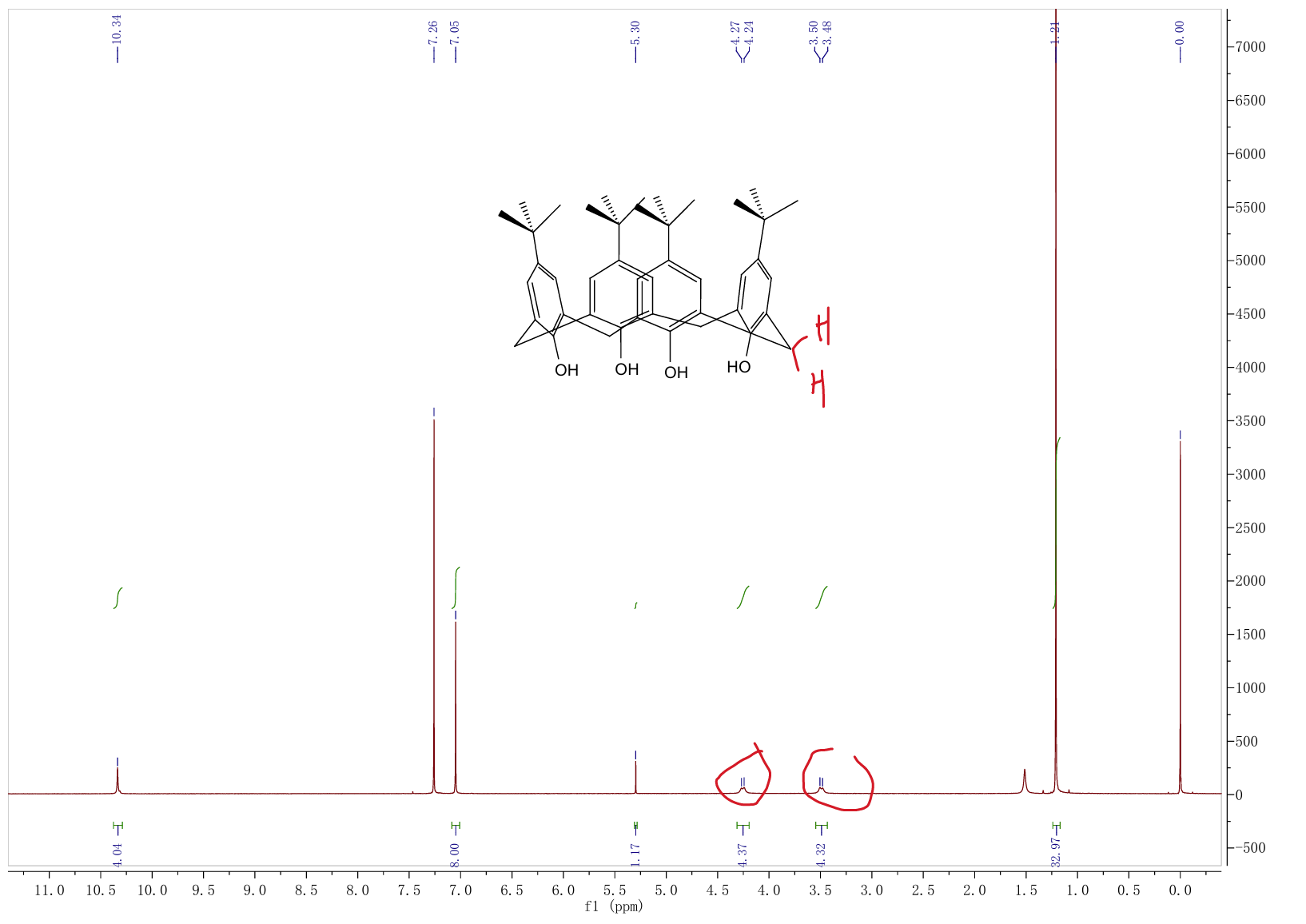
Figure 6: Nuclear magnetic resonance of tert-butylcalix[4]arene compound shows its stable cup-shaped image
The tert-butylcalix[4]arene etherified forms p-tert-butylcalix[4]arene tetraethoxycarbonyl methyl ether (CAS 97600-39-0) (see Fig. 7), and most of this ether molecules remain "cup-shaped" without interpenetrating in solution at room temperature (Fig. 8).
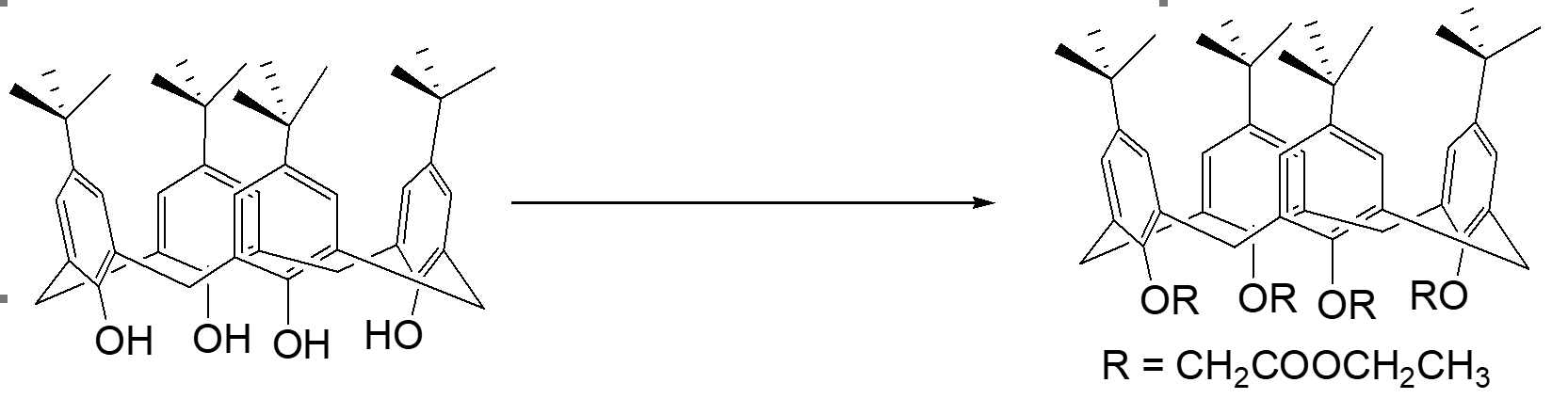
Figure 7: Etherified ethoxycarbonylmethoxy derivative of calix[4]arene compound
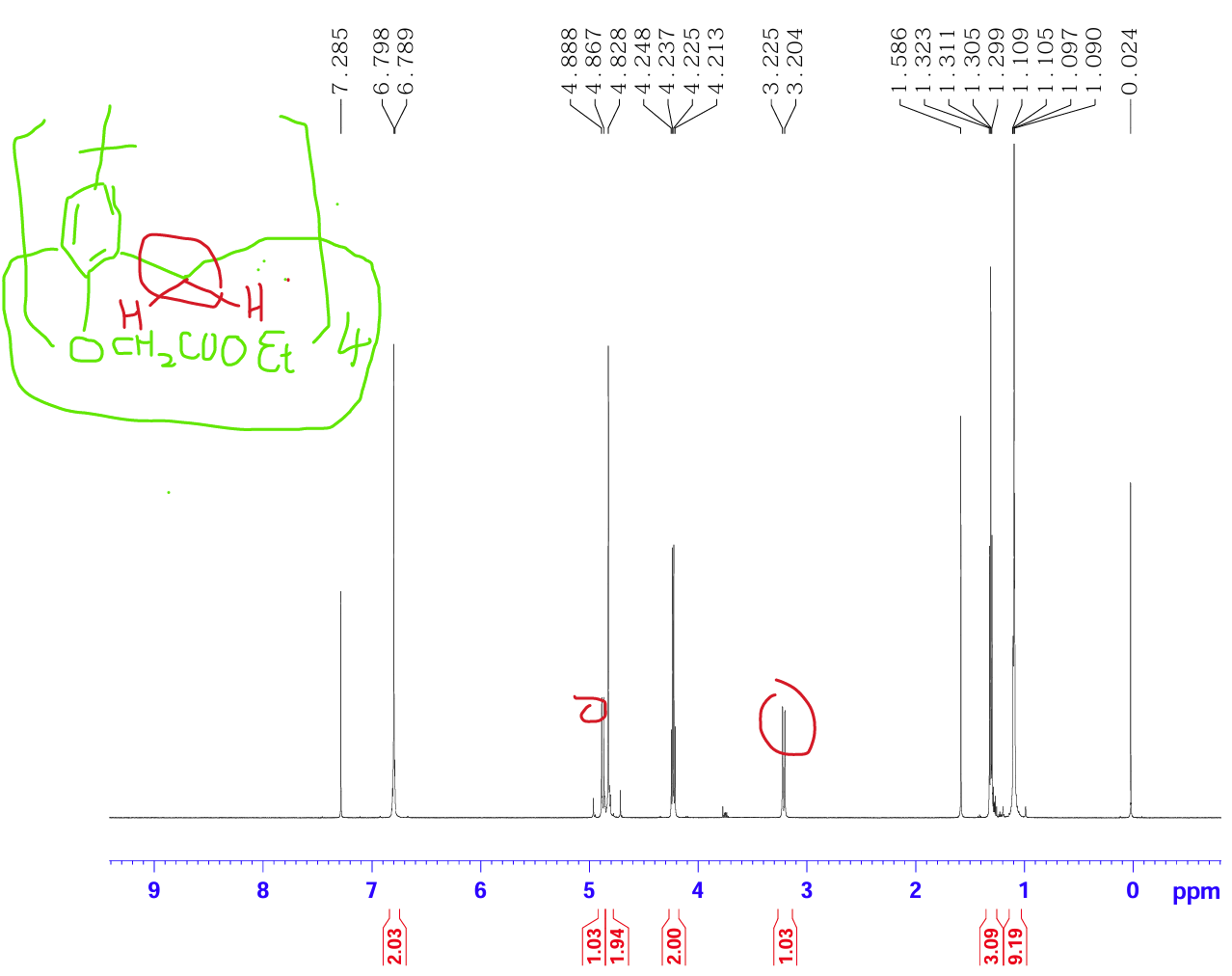
Figure 8: Nuclear magnetic resonance of ethoxycarbonylmethoxy derivative of tert-butylcalix[4]arene compound
In conclusion, the conformation of psychedelic calix[4]arene is not only affected by temperature, but also by the different substituents on the ring, which mainly depends on the substituents. When the substituents on the lower edge are propyl groups or larger groups, the flipping of phenolic hydroxyl groups is hindered due to steric hindrance, and the conformation of calix[4]arene can be fixed. At the same time, the proportion of each conformational variant of the product can also be affected by controlling the reaction conditions, so the target conformation can be achieved by selecting the appropriate reaction reagent, temperature, solvent, etc.
At this point, let's judge the true form of tert-butylcalix[5]arene and calix[5]arene prepared in our laboratory according to the following 1HNMR spectrum (Figure 9 is shown as tert-butylcalix[5]arene, Figure 10 as calix[5]arene). If you have any questions or interesting, please call 13911025849 or Email:bigc.wang@163.com to discuss academic issues together.
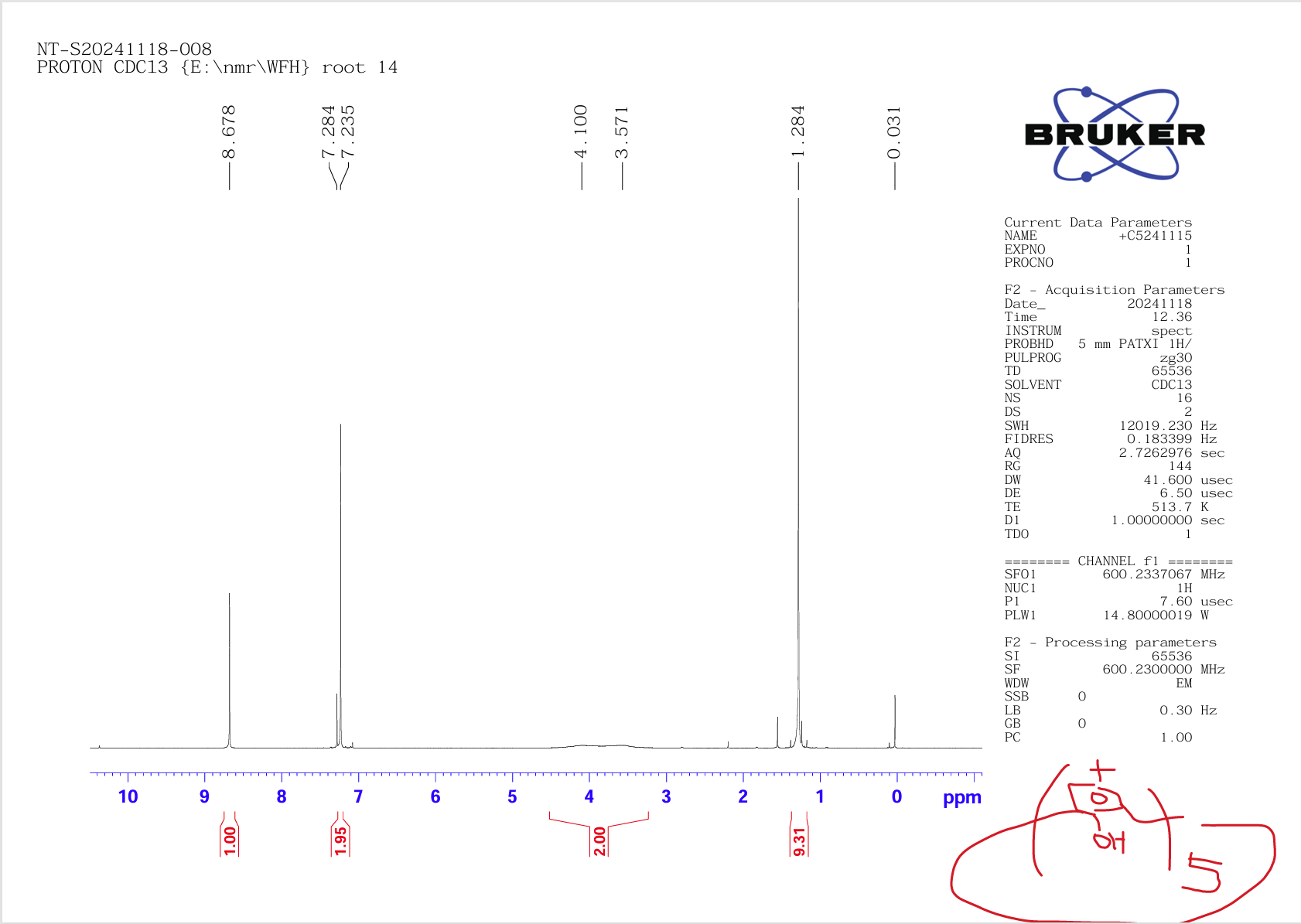
Figure 9: tert-Butylcalix[5]arene compound
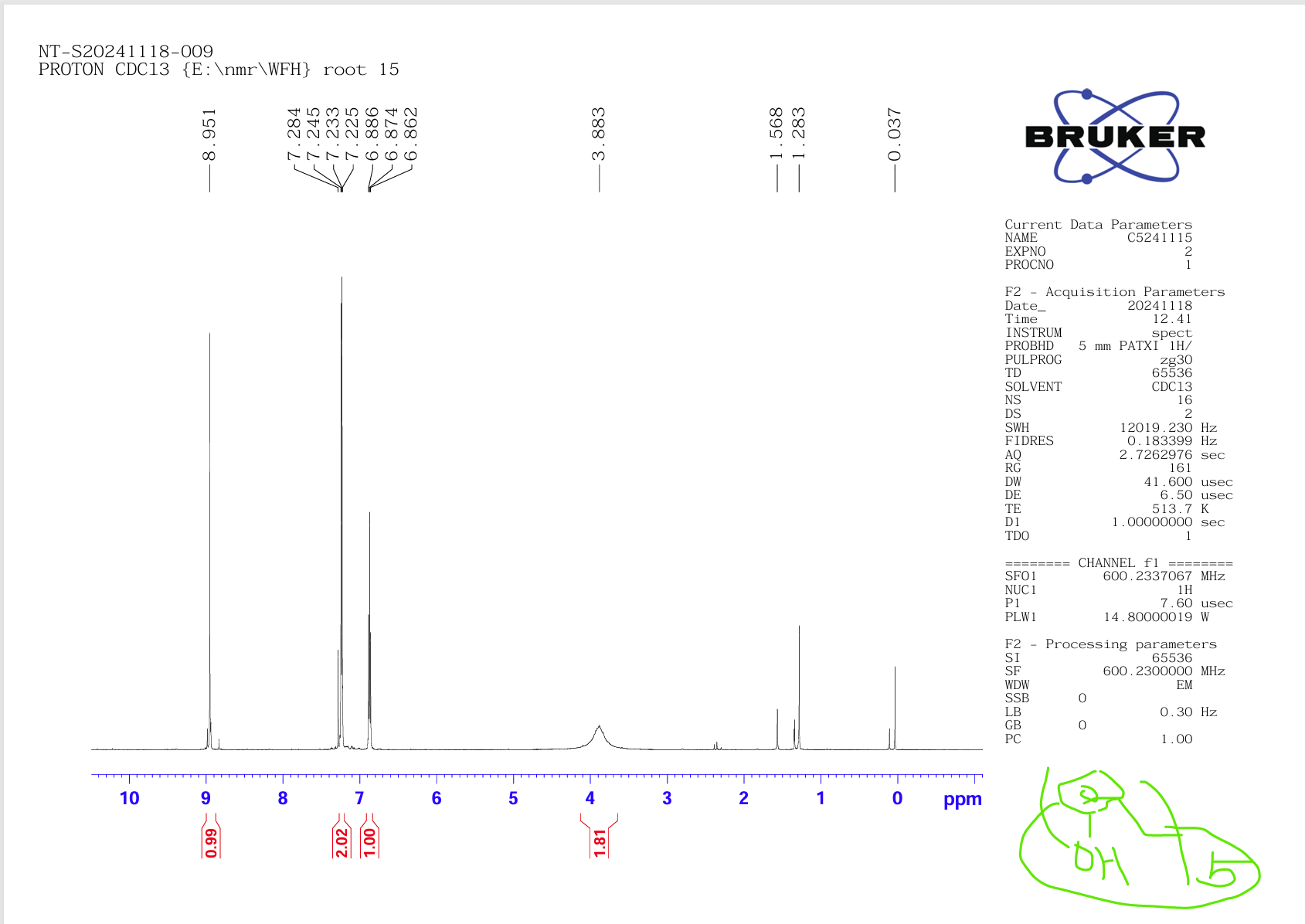
Figure 10: Calix[5]arene compound
Previous:
Next: